Report by Paridhi Agarwal
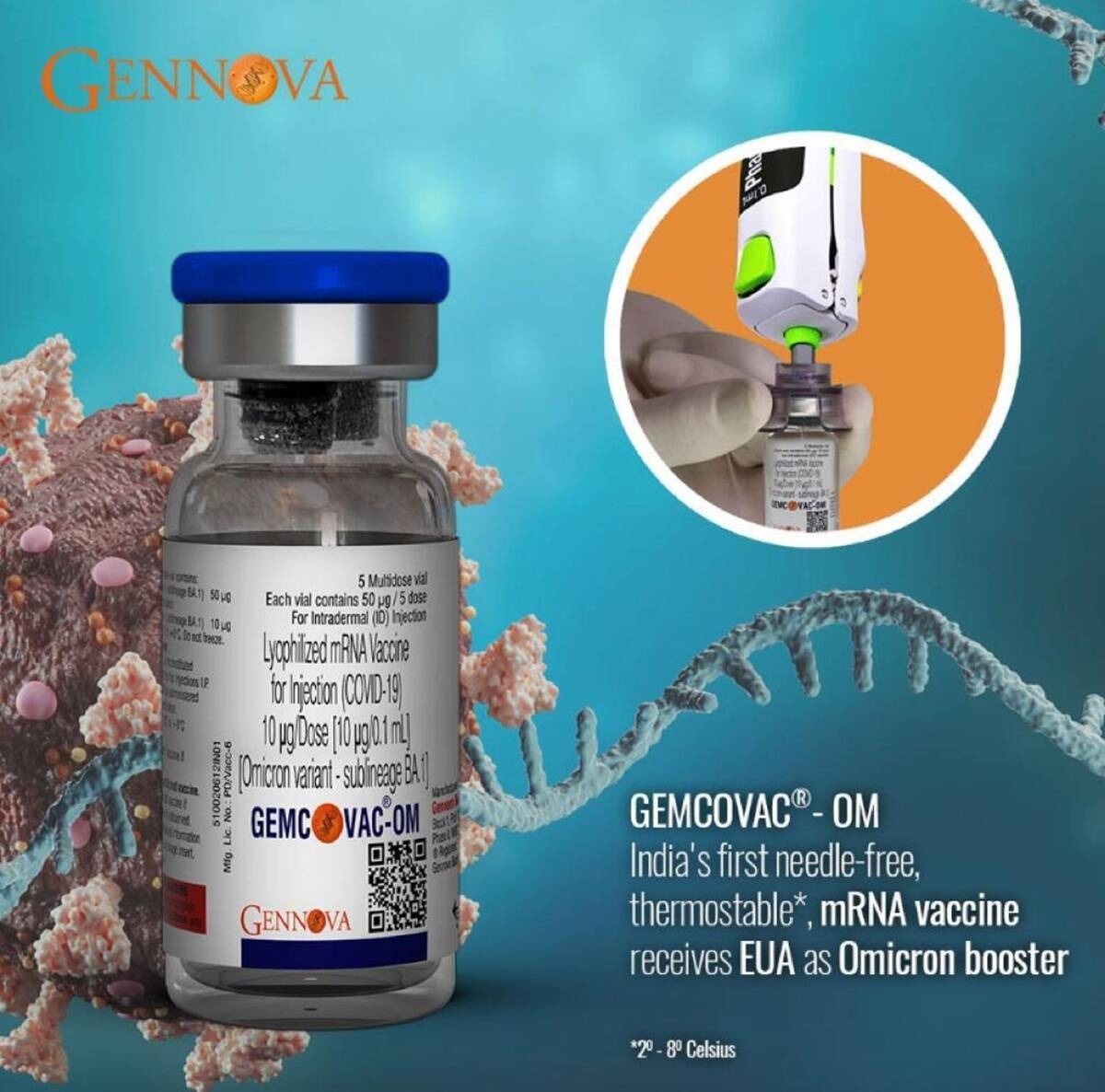
An Indian company called Gennova Biopharmaceuticals Ltd, supported under the Mission COVID Suraksha, implemented by Biotechnology Industry Research Assistance Council (BIRAC), has developed a mRNA booster vaccine specifically for Omicron strain of Covid.
The vaccine has been approved by the Drug Control General of India (DCGI) for Emergency Use Authorization.
The GEMOVAC®-OM is a relatively thermostable vaccine (meaning it is not readily destroyed by heat). It needs a temperature of 2-8℃ for its storage.
The vaccine will be given under the skin using a needle free injection.
Another interesting fact about this vaccine is that for the first time in the country a vaccine has been developed using an indigenous “platform” technology.
What is platform technology?
Older vaccine production technologies have been effective in producing vaccines but are slow and expensive. Typically, the process for developing a viral vaccine using the traditional methods takes 15-to-20-years from the discovery of the virus to the availability of the vaccine in the market. Thus, by the time the vaccine is finally available, the disease outbreak may have caused significant damage.
In order to overcome these challenges in the times of new infections and pandemics, pharmaceutical companies have developed the concept of “platform technology”. In this technology different vaccines can be created using the common base as a “carrier”. Disease specific antigens can then be added on top of this carrier to create vaccines for new infections in a much shorter time frames.
Carriers: Carriers are harmless proteins/ viruses/ bacteria used in the vaccines as vehicles to carry antigens
Antigen: A substance that causes the body to develop a defence system against it. These include toxins, viruses, bacteria, or other foreign substances present in the body.
Advantages and limitations of platform technology for vaccines
Use of platform technologies reduces the time required to develop vaccines and thus help fight the pandemic better. The vaccines developed with this technology also do not need very low temperature to store or transport compared to other vaccines.
However, as this technology is still very new and not a lot of vaccines have been developed using this technology, scientists recommend that more research is needed for the safety and use for these vaccines and in which situations the vaccines need to be used. That is why these vaccines are given Emergency Use Authorization (EUA) only in emergency situations like COVID pandemic.
Emergency Use Authorisation (EUA): Emergency Use Authorization (EUA) is an approval issued for vaccines for use only in a public health emergency. This means that the vaccine has not been fully approved.