A sheet that is exactly one atom thick is called a 2D element.
Let’s understand 2D materials
The first 2D element that scientists were able to create successfully was carbon – in 2004. We know this as graphene.

Several graphene sheets stacked perfectly one on top of another forms granite.
Graphene has many applications in aviation, space, and other domains.
In 2018, MIT created a way to make graphene at scale.
Some transition metals (sulphur, selenium, and tellurium) have also been crafted in 2d structures.
But it was challenging to get a 2D sheet of metals because metals tend to clump up and form nanoparticles when their atoms are spread out, instead of forming a neat, geometrical sheet.
Note: There are many ways to try and isolate one atom thick sheets from materials. You can read about some of them here.
The following steps were taken by scientists at the Linköping University to create goldene:
First, a base material was used in which gold was embedded between layers of titanium and carbon. Silicon was present in thin layers. The idea was to add gold later to make contact.

However, when the team heated the component, they found, to their surprise, that the silicon layer of the base material was replaced completely by gold. This was the discovery of titanium gold carbide.
This meant that they now had a thin layer of gold. The next challenge was to extract it successfully, and to keep it stable.
For many years, the team was unable to extract this gold layer.
Quite by coincidence, Dr. Lars Hultman found out about a reagent called Murakami’s reagent. This chemical is used in Japan in sword and knife making. Among other things, it also removes carbon deposits on steel, making the sword/knife shine brighter.
The team experimented with this reagent for a long time – they changed the temperature and the duration of exposure to see what works best.
They noticed that low concentration over a long period produced good results, but they were still not able to extract the Goldene independently. The last stage was to extract these sheets and keep them stable.

To prevent the exposed two-dimensional sheets from curling up, a tenside was added. A tenside is a long molecule that separates and stabilises the sheets. The scientists described this process as – gold is like cornflakes in a bowl of milk. A sieve is used to put a structure and create the sheets.
What makes goldene special?
When a material is 3D, it forms a layer. When it is 2D, it forms just a coating. With that, its applications change dramatically. Now, it can act as a conductor or a protective coating.
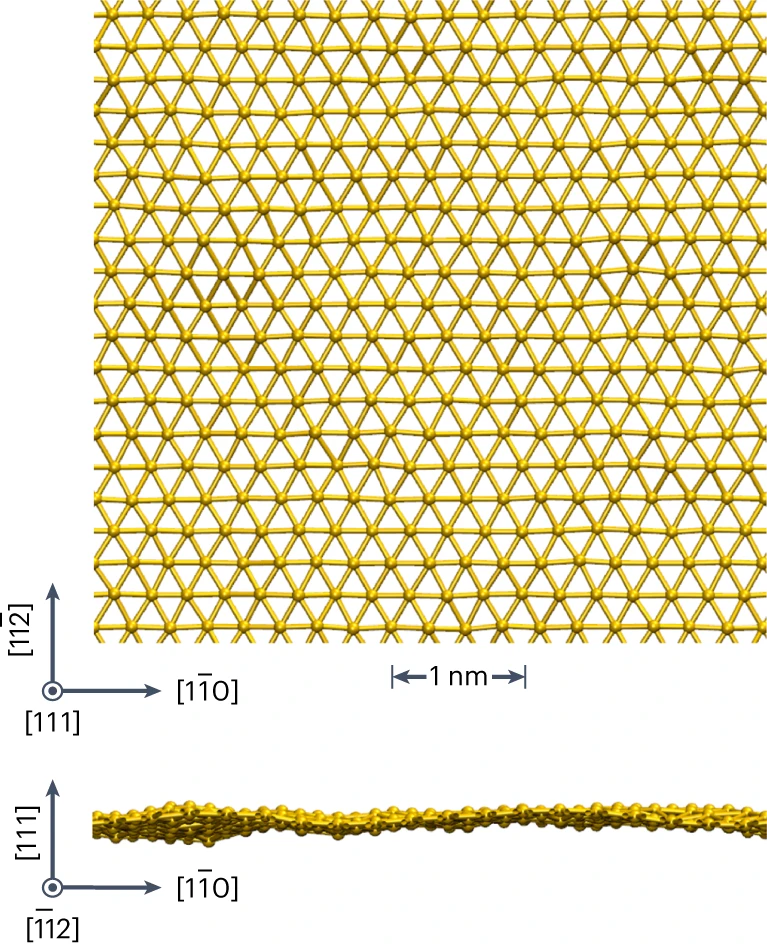
The special properties of goldene are due to that each gold atom has only six neighbouring atoms compared to twelve in a three-dimensional crystal.
While there are many possible applications, at the very least, the use of gold in chips and semi-conductors can be reduced. The same work can now be done using much smaller quantities of gold.
All uncredited images in this article are from the open access paper: https://www.nature.com/articles/s44160-024-00518-4. For all other images/videos, credits as captioned OR given in the video.